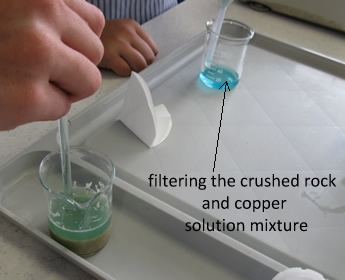
Extraction of copper from its ore.
From ore to awesome.

An ore is defined as a deposit, mainly rock or sediment, that contains a sufficient quantity of a mineral, usually a metal with social and economic importance, that can be economically extracted from the deposit. Extraction usually involves mining and then further purifying until a pure sample of the metal is obtained. Before a deposit is mined scientists test a sample of rock to see if it has sufficient quantities of the required metal to make it’s extraction economically feasible.
In this activity we will use a number of chemical reactions and laboratory techniques to recover pure copper from an ore sample. During these reactions two gases will be produced.
Ore deposits mined for copper typically contain very small percentages of the metal, say around 0.7%. This particular ore, that you are given to test, comes from a place in Australia where the rock is hard to dig into and heavy and expensive equipment is required. With this in mind the ore needs to have at least 2.2%, by mass, of copper metal in it to be economically viable.

Lesson 1
Given a sample of crushed rock containing malechite you are asked to
determine if it is economically feasible to mine this ore.
Construct a photographic flow chart of the procedure you will use.
Document every step clearly and in detail as you will be required
to explain the reasons for your step and any errors that may have
occurred. Use photography to document the steps in your procedure
On the right is the equipment that you are provided with.
- 60 mL from stock solution of 1.0 M HCl (hydrochloric acid)
- funnel + 2 X filter papers
- 2 X 100mL beakers
- glass stirrer
- 8 X 2 cm strips of magnesium ribbon
- electronic balance ( to three decimal places)
- distilled water bottle

insert your pictures in the boxes on the right.
- record the mass of the beaker and sample of ore.
- Is there a chemical reaction taking place? Explain why
- What is the gas given off? How did you identify it? View a video of the gas.
- Describe how the clear acid solution has changed. Explain your
observation.

- Where is the copper in the sand and water mixture?
Give a reason
- Can you decant? Explain why/why not